Too much alcohol in your brew? Taste like vinegar? Where's that sweet/sour balance.? Don't toss it out.
Here is a simply DIY method to adjust the alcohol, acid and sugar level of your probiotic ferment.
note: most people enjoy a well carbonated beverage. What many do not realize that adding carbonation naturally (as in a Second Ferment) is also adding alcohol. The more carbonation - the more alcohol. One mole of sugar is converted to one mole of alcohol and one mole of CO2 (carbonation). If you just want to add lots of carbonation without adding any alcohol that you can do Forced Carbonation.
This method is referred to as Freeze Distillation.
The process works based upon the principle that Water - freezes at a higher temperature (32F) than alcohol, acids (acetic acid, lactic acid, gluconic acid, etc) and dissolved sugar. Most of us know that alcohol does not freeze. (at least in our freezers). CO2, Sugar ,acids all combine with water and lower water's freezing point to around 28F But in our home freezer ICE will form and the majority of the pure clear ice will be water. The slushy colored stuff will be whatever ingredients we added to our probiotic ferment. The very clear portion will be alcohol.
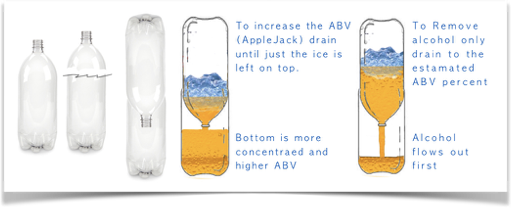
Home Freeze Distiller page 32 Kombucha Craft Brewing Basics.
Freeze a bottle of your brew. Do not fill completely as ice expands and will deform the bottle. Cut the top off one bottle then invert into another bottle. We use 2 - 2 Liter plastic soda bottles. You can repeat the Freeze-Distill process many times to obtain your desired results.
1. Measure the amount you’re freezing.
2. estimate the amount of alcohol.
When your estimate has been drained (into bottle 2 in our example above) you can then decide option 1 or option 2.
Example of how we adjust the alcohol content of our Kombucha
Knowing the amount you started with plus your estimate of that alcohol content (see Determine ABV: SG-FG x 132.715 - TA = ABV) tells you how much to collect or drain off
For example: We have 1 Liter (33.8 oz) of 0.05% ABV Kombucha Tea. That would give us 33.8 x .05 = 1.69 oz of alcohol per liter. As soon as 1.69 ounces collects in the bottom stop the process, and remove the alcohol. hint: pre-mark your collection bottle in increments that are easy to read.
How to calculate the Sugar, Alcohol, and Acid levels of your Ferment.
Testing Sugar, Alcohol & Acids Levels and adjusting flavor.
from Kombucha Craft Brewing 2 nd Edition July 2019 by Ed Kasper HappyHerbalist.com (pages 51-56)
The first thing is to measure the beginning sugar level. Sugar will be converted to alcohol via the yeast, and the acetobacter (bacteria) will convert alcohol to beneficial acids
To use the Hydrometer simply take a sample of your fresh sugar and tea that has just cooled down and add the Kombucha Starting tea and culture, mix and pour a bit into the cylinder. This is taken when you first start your ferment.
Drop in the Glass Hydrometer, give a little spin to remove bubbles
Read across the top of the liquid. Thats the SG (Starting or Original Gravity)
at the top .999. That would be pure water. (1.000)
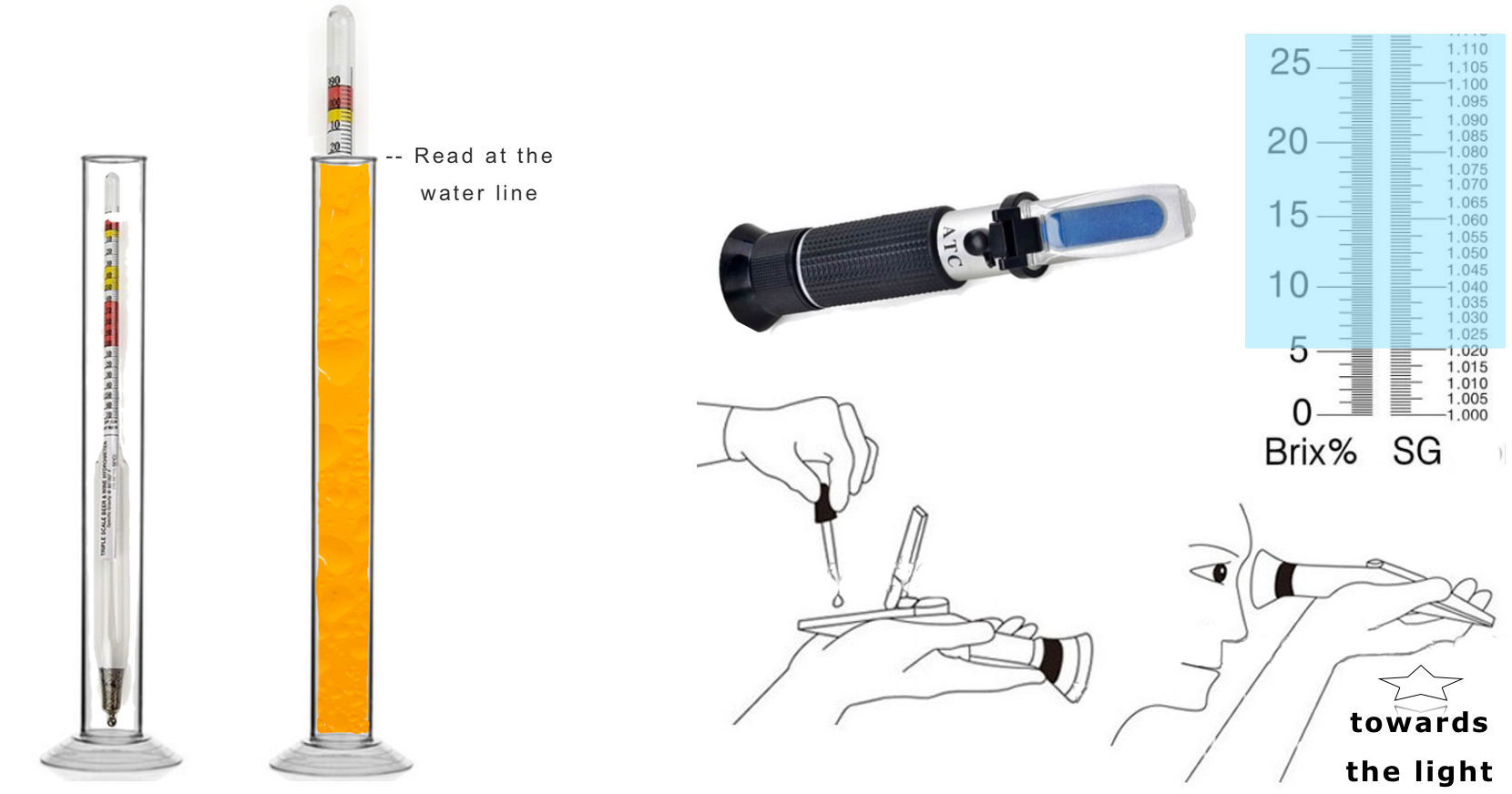
Hydrometer with stand Refractometer Easy way to test sugar levels
Using the Refractometer (above blue box Brix% SG) would be 6% Brix and SG 1.020. Your Starting Gravity (SG) or Original Gravity (OG) is your first reading when you are starting your ferment. Day 1.
Adding more sugar increases the ABV potential. If you want to get more alcohol add 1.5 oz. Sugar x Volume of Liquid / 1 Gallon which will increases the Brix by 1
Adding more water or using less sugar decreases the SG and reduces the potential ABV.
note: Brewing aerobically, cutting off the air supply, such as in bottling, increases the alcohol level as the acetobacter require oxygen to convert alcohol to acetic acid. Cold stabilization - below 42F will cause most yeast and bacteria to go dormant and not produce additionally alcohol or acetic acid.
Take a Second Reading (FG or Final Gravity) when you fell the ferment is finished to your satisfaction - or it just won’t ferment anymore.
Determining ABV (Alcohol By Volume)
- Hydrometer: Take the SG, minus the FG times 132.715 = ABV
- Refractometer Multiply 0.6 times the starting Brix minus the Final Brix times 132.715 = ABV

- For Kombucha Deduct the Total Acids (TA) from the ABV. The reason for this step is that some of the sugar that would have been used for alcohol production is used for acid production. A high acid level will have a harsh tart or acidic taste. If the acid level is too low, will taste flat and bland.
To test the Acid Level, the simplest home testing is using a titration test which can determine the total acids as expressed in Acid Test Titration.

Acid Testing:: Counting the number of drops to change color. or with a pH meter adding drops until the pH reached 8.2
1.Measure 15 cc of brew into the testing vial
2. Add 3 drops of Phenolphthalein into the brew, Cover and shake.
3 .Fill syringe with 10 cc of Sodium Hydroxide. Drop 1 cc at a time into the testing vial and swirl after every drop. You are looking for the point where color change occurs.
4. .Multiply the amount of cc’s of the Sodium Hydroxide you added times 0.10 to determine the acid level. For example if you used 3 cc to effect color change then the acid content is (3 x 0.10 = 0.30%) or 0.30% TA or 3 g/L
note: Acid Test kits allow for about 50 tests.
note2 for dark colored brews dilute with distilled water and hold the test vial up to a wide white background. Distilled water is neutral so the amount added does not matter.

To determine the Potential Alcohol in kombucha subtract Total Acids (TA) from the Potential Alcohol (PA), and that should leave you with a workable alcohol level to help know your basic brewing results for a home brewer. Just like a Home Beer / wine brewer these are estimates and not acceptable for the TTB which requires a more sophisticated and expensive determination. If you should decide to have a few batches tested accordingly that would give you a margin or error that you could work with as a benchmark.

Adjusting Alcohol, Acids, pH and Sugar levels.
At times you may want to reduce or increase these levels. There are 2 simply formulas available. You can mix different ferments (with known values) or use water, or water mixed with adjuncts.
1. Aa+Bb=Cc
Capital letters express quantities and lowercase express properties.
Let's say I have 640 oz (5 gallons) of Kombucha Tea at 1.5% ABV . How much ABV will I have if I add 320 oz (2.5 gallons) of water?
A=640 oz. a= 1.5 ABV = 960
(960)+(320X0) = 960
640 + 320 + 960
960 / 960 = 0.0 ABV
Of course you’ve watered down your Kombucha Tea both in alcohol, flavor and acids. But instead of watering down with plain water, you could add flavor, sugar* and/or acids to the water to achieve the balance you’re looking for. The same formula may be applied to pH, sugar*, acid levels.
- Avoid adding flavors or sugars that are fermentable as results will then vary accordingly.
2. Persons Square
V₁× n₁ = V₂× n₂,
where V: volume and n: the number of moles of solute in the solution (obviously, the same holds true for any similar quantity, such as alcohol by volume, i.e. you can replace n with ABV%). Free online calculator www.prechel.net/formula/pearson.htm
Pearson Square Using a Sweet Kombucha to Balance a Too Sour Kombucha Or how Home Wine Makers Balance their wines.
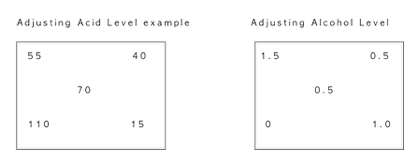
In the example above blending Kombucha Tea with two different acid levels, one being .55% TA the other 1.10% TA, we can use the Pearson Square to determine the ratio needed to obtain any desired acidity level between .55% and 1.10%. In this example we a shooting for an acid level of .70%. Adjusting alcohol level from 1.5 to 0.5 shown on the right.
The top left corner and the bottom left corner represent the acid level of the two kombucha teas to be blended. The center number is the acid level we want to achieve. The two numbers on the right are numbers that you calculate. The 15 is the difference between 55 and 70, and like wise the 40 is the difference between 110 and 70.
The 15 and the 40 represent the blending ratio of the kombucha teas that will result in the desired acid level; 40 parts of .55% wine and 15 parts of 1.10% wine. (Reduce to common divisor of 5 to 8 parts and 3 parts respectively).
All ferments are affected by the pH, Optimum pH for both the yeast and bacteria is around 5.4. and a good starting point for growth. Fermentation slows as the pH drops towards 2.5 where it pretty slows or stops altogether. To preserve foods requires the pH to be 4.6 or below. Molds and pathogens may however grow all around or on top of ferments even though their (liquid) is well below 4.6. Mold is a greater threat to kombucha due to the aerobic nature of kombucha brewing. A good starting pH for kombucha is 4 to 4.5, which allows for good mold protection and good environment for the kombucha bacteria and yeast to thrive.

Beer can finished at a pH 3.5 to 4. while Kombucha Tea’s pH is in the range of 2.5 to 3.5. For preserving your ferment pH must be < 4.0
A Secondary Ferment the acidity may first need to be raised in order for any yeast to go to work. Baking Soda will quickly neutralize acids but too much leaves that baking soda aftertaste. Calcium Carbonate is a great healthy solution first mix in warm water as its not too water soluble, We use our mini-kegs CO2 Carbonator, as the calcium breaks down under CO2 and the small amount of CO2 benefits the yeasts.
Stop the Primary Kombucha Tea Ferment when the pH is above 3.7 and the Final Gravity is above 1.010. The higher the pH and the higher the SG the greater the ABV.
When bottling and planning on storing for later, the pH of your kombucha brew - at time of bottling - the pH should be under 4.0.
Now most foods and universities and the FDA, state that a pH under 4.6 is required for foods to be considered safe. That is when bottled and pasteurized per their safe food handling guidelines. However we feel that since most all kombucha ferments start out over 4.0 pH and unless the kombucha begins to ferment - as it may not in all cases - mold may develop or the kombucha has probably died and should be discarded. Typically a Kombucha ferment will go to a pH between 2.5 to 3.5 in 8-14 days when fermented at a temperature range of 74-84F.
- from our Kombucha Craft Brewing Guide available as a free PDF Download we also offer Kombucha Mushroom Extracted, our approach to using the Kombucha mushrooms (SCOBY) in various forms such as an extract, distillation, and concentration. With detailed information on Do It Yourself https://www.happyherbalist.com/books/
For more information refer to our article online "How to Brew Alcohol Free Kombucha Tea " and be sure to check out the Alcohol levels tested on top commercial kombucha tea producers from a 2017 study.

